In honour of Breast Cancer Awareness Month this October, Rudi Van Den Eynde, Head of Thematic Global Equity, gives us an overview of the disease and the challenges created by the epidemic. He is pleased to see that patient care is getting back to normal in hospitals, and that pharmaceutical innovation is still going strong, driven by better understanding of the disease.
Breast Cancer Awareness Month has taken on an all-new meaning this year with the advent of the Covid-19 crisis. What impact has the pandemic had on the diagnosis and treatment of patients?
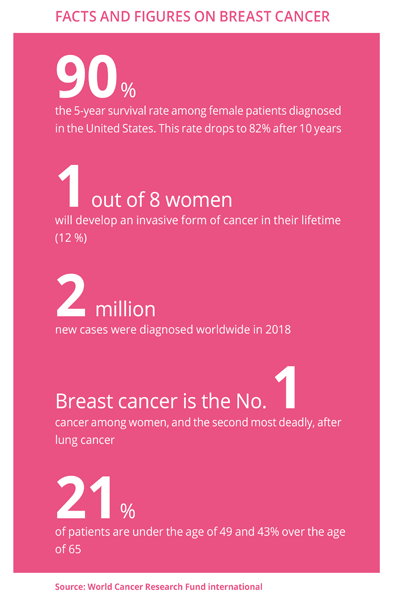
We don’t yet have the exact numbers, and probably never will, but the first few months of the pandemic significantly disrupted cancer treatments. There was a major panic, during which many patients preferred to stay at home rather than seek or keep an hospital appointment, operations were cancelled, and most importantly testing declined. Healthcare services have since got back on track. Sick patients can be seen in completely safe conditions. Of course, the situation is still complex, and some procedures may still be delayed, but testing campaigns are being resumed at a normal pace. And let’s not forget that such campaigns are critical in order to diagnose and treat cancer at a very localised stage, with very high cure rates. Inclusion of patients in clinical trials is also on the rise and almost back to normal. That’s very good news for research, and for all patients without the option of alternative therapies, who can thus benefit from the latest innovations.
There is not just one type of breast cancer. In fact there are many different types.
Could the priority placed on coronavirus research slow progress in oncology?
No! It is clear that, right now, there is greater focus on infectious diseases. Many labs are developing vaccines and treatments against Covid, but these are also the same companies already specialising in this field. There are more public- and private-sector funding operations, but they are not taking anything away from oncology or any other disease. The healthcare industry needs a full pipeline to prepare for the future and will not delay its promising treatments. Nor does it lack the cash flow it needs to keep research moving forward on multiple fronts. Furthermore, there is no skills transfer: the experts working on the coronavirus are not from the oncology field. It would be impossible to implement such a transfer. Even companies boasting cutting-edge expertise in oncology are not going to change their focus in just a few months.
The healthcare industry needs a full pipeline to prepare for the future That said, such projects will not delay treatments already under development.
What is the most promising scientific work being done?
Research now offers a better understanding of the genetic signature of different types of cancer, opening the door to personalised medicine and the development of highly specialised drugs. Roche’s Herceptin® was the first step in this revolution, transforming the prognosis for HER2-positive breast cancer, one of the most aggressive forms of this cancer. On the downside, it is effective only on 20% of patients bearing this gene mutation. For others, however, new alternatives are gradually replacing, or supplementing, chemotherapy treatment. Recently, the FDA (US Food & Drug Administration) approved a new drug for “triple negative” cancer, one of the most complex forms of breast cancer to treat. Hereditary BRCA cancer treatments have also improved, as have treatments for cancers that have advanced to the metastatic phase or are no longer responding to standard treatment. Promising research is also being done in immunotherapy, with the goal of stimulating the immune system to better address tumours.
 The cost of such innovative treatments is climbing fast, however, and is even skyrocketing for some, with the risk of making them harder to access...
The cost of such innovative treatments is climbing fast, however, and is even skyrocketing for some, with the risk of making them harder to access...
That’s the cost of targeted medicine. Until the early 1990s, new treatments primarily addressed large patient populations. Cancers were treated as effectively as was possible with chemotherapy. Since then, however, research has shown that there is not just one type of breast cancer. There are actually many types that can, and should, be treated with different drugs. Generally speaking, in oncology, some very rare cancers only affect a few tens of thousands of patients around the world, and sometimes just hundreds... Biotechnology has given us weapons to fight them, but that also means that each drug that is developed can only be prescribed to a limited number of patients. Meanwhile, R&D costs have risen incessantly, forcing prices up in an increasingly fragmented market. Of course, we can’t be naive, and negotiations between healthcare authorities and labs are critical to regulating prices, as is the intense competition waged between labs. What’s more, we must not forget that putting drugs on the market creates funding for the innovation of tomorrow. And, when patents expire 10 to 12 years after they are approved for market, competition from generic and biosimilar drugs becomes very strong and prices drop. Ultimately, I find the system fairly efficient and think it stimulates the investments needed to meet future challenges.
Private-sector investors also have a role to play in this system, by funding the most promising projects. Are they still showing interest?
If we look to the past, in venture capital, there were certain voids not being filled, and companies had to engage in fierce competition to attract investors, especially in Europe. When coronavirus came along, it seemed like venture capital might get “crowded-out” towards other treatment areas. That wasn’t the case, however, which just goes to show how expansive research is today and how promising are innovations for the future. Lastly, I would say that venture capital in the healthcare industry is not only gaining ground in most European countries, but also improving. As a general rule, companies have the necessary funds to do their work. Those with the right innovation have no trouble in that department. I would almost venture to say that, in some countries, there is more capital than big ideas. Which is why is it also important for investors to conduct an in-depth analysis of the projects they plan to invest in, no matter what the type of company (publicly traded or private), by relying on renowned experts. That’s what we at Candriam offer, with our team of investment-savvy scientists, which have proved their worth for nearly 20 years now, even in an environment as complex as that created by the coronavirus.
